Download the A&E Case Report Form
Any episode of a significant allergic reactions (not just anaphylaxis) occurring in the community following unintended allergen exposure (irrespective of whether the trigger is food), can be entered into the Registry with an emphasis on those with features of anaphylaxis.
Where the clinician is confident that no allergen exposure is likely to be involved e.g. idiopathic urticaria, then there is no need to report. The priority for reporting is:
The accidental allergic reaction MUST have occurred within the last 12 months and not have been caused by food/drug challenges.
Please remember that verbal consent and email address for the patient must be sought to be able to have access to the Case Report Form (CRF) and this must be done by the members of the active clinical team that liaise with potential patients in the first instance.
If someone who is a member of the research team (or indeed, a different clinical team not involved in that patient’s care) contacts the person who had the allergic reaction (or their parent/carer etc), then they do not have legal consent to access that person’s details and would therefore be breaking data protection legislation.
The Registry has a dedicated CRF for allergic reaction. This CRF enables HCPs to record any (child or adult) episodes of severe allergic reaction in the community following unintended allergen exposure. An abbreviated version of the CRF has been also developed to match the needs of staff working in A&E and GPs, specifically. If you are already registered you can report a case. If you are already registered click here to report a case.
Paper versions of CRF and A&E CRF can also be used to record case details, which can be entered into the Registry platform by local staff later. The paper versions are available at A&E CRF paper version and CRF paper version and can also be downloaded from the Registry “Document” section.
The Registry is included on the NIHR-CRN portfolio. For each completed CRF, the local centre will receive accruals. The Registry team will log cases centrally on CPMS.
The UK Anaphylaxis Registry has been approved as a National Research Database by the NHS HRA. This means that HCPs can register without the need for local research approval or specific contracts. HCPs may wish to confirm with the local R&D departments if they wish to review study documents and guidance, in which case these are available at UK Anaphylaxis Registry: REC documents
Verbal consent needs to be obtained from patients/families. Individuals should make sure they have undergone appropriate training (e.g. GCP, local study-specific training) for this purpose.
You must register prior to reporting any cases. Any HCP working in hospital setting can register. There are two options:
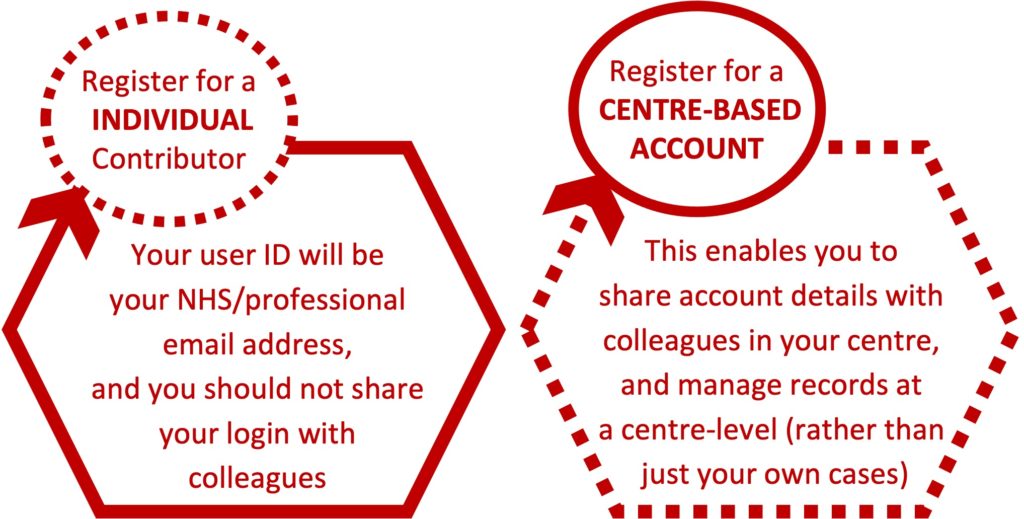
To request a centre-based account, you must be a medical consultant or Band 7+ nurse. You should register as an individual but request a Centre-based account when registering. We will send you a Centre User ID and password which you can share at your discretion.
Once your account has been approved, we will email you with your User ID and Password, to gain access to the online platform.
Click here to register or scan the QR code below.
