October 4th Update on the position of Emerade Auto-Injectors following a review from the MHRA’
EMERADE 150/300/500 MICROGRAMS SOLUTION FOR INJECTION IN PRE-FILLED PEN- COMPLAINTS OF INITIAL FAILURE TO ACTIVATE
Dear Healthcare Professional,
PharmaSwiss Ceska Republika s.r.o. (an affiliate of Bausch and Lomb UK Limited), with the agreement of the European Medicines Agency (EMA) and Medicines & Healthcare products Regulatory Agency (MHRA), wishes to inform you of the following in relation to Emerade 150/300/500 micrograms solution for injection for treating severe acute allergic reactions (anaphylaxis):
Summary
- The MHRA has notified Bausch and Lomb UK Limited of reports that Emerade pens have failed to activate. This is not related to the risk of needle blockage reported in the previous drug alert; This risk of needle blockage is expected to be resolved in all new stock manufactured since July 2019
- The activation failures have not been traced to particular batches. Bausch and Lomb UK Limited are conducting a thorough investigation to identify the reason.
- Following discussions with alternative adrenaline auto-injector suppliers there are currently sufficient supplies available to meet historic demand. However, there are insufficient surplus devices to replace all the Emerade pens that would need to be recalled.
- Therefore, at present, the MHRA is advising that Emerade devices should not be recalled.
- On the basis of all the information available, most pens will activate as normal.
- Patients are advised to continue to follow existing advice to carry two in-date pens with them at all times.
- When an Emerade pen is used, it should be pressed very firmly against the thigh. If this does not result in activation, the patient should immediately use their second pen. Information is provided to patients on page 5.
- If the patient is not improving, suggesting a further dose of adrenaline is needed, additional attempts should be made to administer a pen that has failed to activate, while awaiting the arrival of the emergency services.
- Reporting
Activation Issue – Further Information
An activation failure means that the needle is not released from the device and therefore the injection is not administered. Bausch and Lomb UK Limited are conducting extensive investigations. It has been confirmed that some Emerade pens did not activate when normal force was applied, however, the rate of occurrence cannot be accurately estimated at present.
Supply
Following discussions with alternative adrenaline auto-injector suppliers, there are currently sufficient supplies available to meet historic demand. However, there are insufficient surplus devices to replace all the Emerade pens that would need to be recalled. Therefore, at present, the MHRA is advising that Emerade devices should not be recalled.
The MHRA advice, endorsed by the UK Commission on Human Medicines, is that in the interests of patient safety it is important to allow patients to keep their Emerade pens at present so they still have access to adrenaline instead of risking many patients being left without any adrenaline pens. It is emphasised that the majority of Emerade pens in circulation will activate and deliver adrenaline as expected. However, it is important for patients to be aware of the possibility of activation failure so they can take measures to ensure they always have two pens with them and to be aware how to check that a pen has activated successfully.
In the UK there are two alternative adrenaline auto-injector devices on the market, EpiPen, manufactured by Mylan and Jext, manufactured by ALK-Abello. These different brands of adrenaline auto-injector are not used in exactly the same way so specific training and advice for patients and carers is required before using each of the devices.
The MHRA is fully aware of the issues and is being kept informed by Bausch and Lomb UK Limited of their ongoing investigations.
It is important to report all suspected adverse reactions or product quality defects via the Yellow Card Reporting tool, https://yellowcard.mhra.gov.uk/ More information can be found on page 5.
Needle Blockage – Further Information
The previous notification related to a risk of needle blockage, that comes with a very small risk of inadvertent injection of small particles released from the blockage into the bloodstream. There would be a risk of a minor inflammatory response to a once only injection of particles into muscle or subcutaneous tissue.
This risk is expected to be resolved in all new stock manufactured since July 2019 that will now start to be released to the market. It will take some time before the new stock can replace all the existing stock in circulation.
The activation failures are not specific to any particular batches of product and are not related to the potential needle blockage.
Information for Patients:
The MHRA has provided additional information below (see pages 4 and 5) for patients and carers who have Emerade pens. Information is provided on the activation failures and advice on how to handle an emergency event should a pen fail to activate and how to tell whether this has occurred by inspection of the pen.
Healthcare professionals are urged to share this information with all patients and carers who have been prescribed an Emerade pen.
Dear Emerade Adrenaline Autoinjector Users:
The Medicines & Healthcare products Regulatory Agency (MHRA) has received reports of Emerade pens failing to activate. This means that the needle is not released, and the injection is not delivered.
This is different from the needle blockage issue that you are likely to have been informed about previously by your healthcare professional via a drug alert. This risk is expected to be resolved in all new stock manufactured since July 2019 that will now start to be released to the market.
The cause of the activation failures is being intensively investigated but is currently unknown.
Following discussions with alternative adrenaline auto-injector suppliers there are currently sufficient supplies available to meet historic demand. However, there are insufficient surplus devices to replace all the Emerade pens that would need to be recalled. Therefore, at present, the MHRA is advising that Emerade devices should not be recalled.
The activation failures have not been traced to any particular batches of product.
The MHRA understands that you will be very concerned that the pens in your possession may be affected. It is important to note that based on all the information to date, the majority of pens will activate as normal.
If you do need to use an Emerade pen, as a precaution you should press the pen very firmly against the thigh. If this does not result in activation, you should immediately use your second pen. Information is provided below/on page 5 to illustrate what a non-activated pen looks like.
If a further dose of adrenaline is needed before the emergency services arrive, additional attempts should be made to administer a pen that has failed to activate. This is because reports received suggest that a pen may activate after further attempts. You are reminded to carry two in-date pens with you at all times.
For more information, please refer to the patient information leaflet. There is also a fact sheet with advice on the use of adrenaline auto-injectors which you and your carers are encouraged to read.
A full investigation is ongoing. The MHRA and Bausch and Lomb UK Limited will provide updated information to healthcare professionals and affected members of the public as soon as it becomes available.
You can help us by continuing to report any issues directly via the Yellow Card reporting tool, https://yellowcard.mhra.gov.uk/
Download the free Emerade mobile app. The free Emerade mobile is available for iPhone and Android phones and includes:
- Instructions for Emerade
- Symptoms of anaphylaxis
- Video demonstration
- Reminders to carry Emerade at all times
- Download the app now by visiting the App Store or Google Play and search for “Emerade”.
Remember; if you are moved onto a different brand, you must ensure you receive adequate training from your healthcare professional as each product is used differently.
WHAT DOES MY EMERADE PEN LOOK LIKE BEFORE USE? Fig. 1

BEFORE USE
Instructions:
1. An unused Emerade pen, with front cap in place (Fig. 1).
2. For instruction on how to use your Emerade pen please consult the Patient Information Leaflet (PIL).
3. During this period, when activation failure is a possibility, you should press the Emerade pen very firmly against your thigh.
HAS MY EMERADE PEN ACTIVATED? Fig. 2

ACTIVATED
When Emerade Pen has been activated the needle cover will extend and lock.
Instructions:
1. After using an Emerade pen following the instructions found on product labelling, verify that the pen has activated.
2. An Emerade pen that has been activated, will have an extended needle cover (Fig. 2 – circled section of image)
3. Call 999 for an ambulance and state “Anaphylaxis” even if you start to feel better
4. Lie flat with your legs up to keep your blood flowing. However, if you are having difficulty breathing, you may need to sit up to make breathing easier
5. Proceed to administer your second pen if you are not improving in case you need a second dose of adrenaline
WHAT DO I DO IF MY EMERADE PEN HAS NOT ACTIVATED? FIG. 3
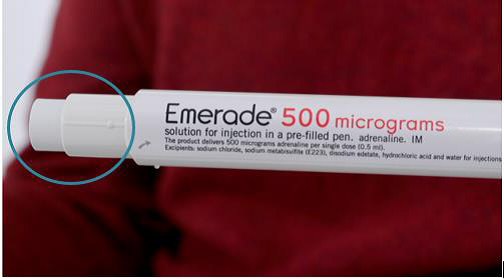
NOT ACTIVATED
If the needle cover has not extended, the pen has not activated.
Instructions:
1. If the needle cover has not extended, the pen has not activated (Fig. 3 – circled section of image).
2. If the pen has not activated, use your second pen immediately.
3. Call 999 for an ambulance and state “anaphylaxis” even if you start to feel better.
4. Perform additional attempts to activate, if pen(s) fail to fire initially and further dose is needed. This should only be attempted once all pens have been tried.
5. Retain any suspected, un-activated pen for reporting to the MHRA via the Yellow Card (further information on page 5) and investigation purposes.
Call for reporting
The reporting of suspected adverse reactions is of great importance. It allows continuous monitoring of the benefit-risk balance of the drug. Healthcare professionals and patients are encouraged to report any suspected defect or adverse event to:
Please continue to report suspected adverse drug reactions (ADRs) to the MHRA through the Yellow Card Scheme.
Please report:
- all suspected ADRs that are serious or result in harm. Serious reactions are those that are fatal, life-threatening, disabling or incapacitating, those that cause a congenital abnormality or result in hospitalisation, and those that are considered medically significant for any other reason
- all suspected ADRs associated with new drugs and vaccines identified by the black triangle▼
It is easiest and quickest to report ADRs online via the Yellow Card website –
https://yellowcard.mhra.gov.uk/ or via the Yellow Card app available from the Apple App Store or Google Play Store.
Alternatively, prepaid Yellow Cards for reporting are available:
- by writing to FREEPOST YELLOW CARD (no other address details necessary)
- by emailing [email protected]
- at the back of the British National Formulary (BNF)
- by telephoning the Commission on Human Medicines (CHM) free phone line: 0800-731-6789
- by downloading and printing a form from the Yellow Card website (see link above)
When reporting please provide as much information as possible, including information about medical history, any concomitant medication, onset, treatment dates, and product brand name.
Contacts for Further Information:
For stock enquiries please contact Bausch & Lomb Customer Services, Tel: 0208 781 2991
Email:[email protected]
For medical information enquiries please contact the Pharmacovigilance and Medical Information Officer, Tel: 0208 781 5523, Email: [email protected]
Further information on Emerade including use of the app can be found at https://www.emerade-bausch.co.uk/
Recipients of this communication should bring it to the attention of relevant contacts by copy of this letter. NHS Regional Teams are asked to forward this to relevant clinics, general practitioners and community pharmacists for information / action.

Jenni White
Business Unit Head Pharmaceuticals & GDP RP
UK/EME/19/015 Date of Preparation: October 2019
| Visioncare | Surgical | Pharmaceutical | Aesthetics |
| T 0845 602 2350 | T 020 8781 0000 | T 020 8781 2991 | T 0845 600 5212 |
| F 0845 602 2351 | F 020 8781 0001 | F 020 8781 0001 | F 0845 600 5215 |
Registered Office: 106 London Road, Kingston-upon-Thames, Surrey KT2 6TN Registered in London Number 143720
